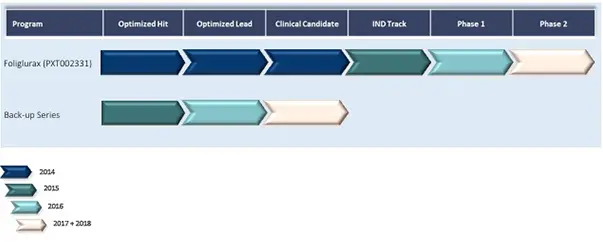
Prexton’s mGluR4 Pipeline: Foliglurax as a First-in-Class Therapy for Parkinson’s Disease
Prexton Therapeutics has built a promising pipeline focused on New Chemical Entities (NCEs) that selectively target the metabotropic glutamate receptor 4 (mGluR4). This innovative strategy aims to provide a novel symptomatic treatment for Parkinson’s disease, moving beyond traditional dopaminergic therapies such as L-DOPA, which often lead to serious long-term complications like motor fluctuations and dyskinesias.
The company’s lead compound, Foliglurax, represents the first-in-class molecule designed to stimulate mGluR4 activity. By modulating this receptor, Foliglurax is expected to restore balance in basal ganglia circuits and alleviate motor symptoms such as rigidity, tremors, and dyskinesia. Importantly, this approach does not directly act on the dopaminergic system, thereby holding the potential to reduce side effects and prolong treatment benefits for patients.
Having demonstrated encouraging efficacy and safety results in preclinical studies, Foliglurax advanced into Phase II clinical trials in 2017. These studies are designed to evaluate its ability to reduce “OFF” periods (times when medication is not working effectively) and to control Levodopa-Induced Dyskinesia (LID), one of the most disabling complications of current therapies. If successful, Foliglurax could become a game-changing therapeutic option for millions of patients worldwide living with Parkinson’s disease.